Enhanced TDS
Identification & Functionality
- Carrier
- INCI Name
- Manufactured By
- Cosmetic Ingredients Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Labeling Claims
- Function
GreenGard™ L is a cutting-edge, 100% biobased, multifunctional ingredient that offers a host of benefits for skincare and haircare formulations. In-vitro testing has confirmed the ability to provide additional skin advantages, such as reducing TEWL and upregulating collagen I production. This natural, organic acid preservation systems exhibit their utmost efficacy within a pH range of 4.5 and 5.5. GreenGard™ L can be paired with an antifungal for comprehensive preservation.
- Product Benefits
- 100% Biobased
- Multifunctional antimicrobial ingredients
- Additional skincare benefits with addition of arginine
- Challenge data available for variety of product types
Applications & Uses
- Markets
- Applications
- Application Format
- Personal Hygiene Applications
- Wipes, Tissue & Towel Applications
- Use Level
- 1.5 - 2.5%
- Application pH Range
- 4.5 - 5.5
- Formulation Guidelines
- Can be combined easily with other organic acids if needed
- Effective performance is given at pH range 4.5 and 5.5
- Can be incorporated into hot or cold process formulations
- Formulating Guidelines
pH Optimum 4.5 - 5.5 Use level 1.5 - 2.5% Claims 100% USDA Biobased, Vegan, non-GMO, PEG-free, TEWL reduction, and Collagen I
upregulationUses Toners, lotions, creams, balms, and wet wipe solutions
Efficacy Spectrum

Properties
- Physical Form
- Odor
- Characteristic
- Appearance
- Colorless amber liquid
- SDS Physical and Chemical Properties
Value Units Test Method / Conditions Form Liquid - - Initial Boiling Point and Boiling Range 245 °C - Vapor Pressure (at 102°C) 1 hPa - Relative Denslty 1.156 g/cm³ - - Specifications
Value Units Test Method / Conditions Specific Gravity (at 20°C) 1 - 1.2 - GLTM002 Refractive Index (at 25°C) 1.4000 - 1.5000 - Refractometer pH Value 4 - 6 - GLTM003 - Note
This product contains natural ingredients. There may be color & odor variations from lot to lot due to crop fluctuations from harvest to harvest.
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- GreenGard™ L TEWL Study
Objective: To compare the skin barrier protective properties of GreenGard™ L , an organic acid arginine salt, against those of an organic acid ionic salt competitor through in vivo evaluation of Trans-epidermal Water Loss (TEWL). Study Design: Skin barrier protective properties were quantified through the reduction of TEWL on human skin. TEWL readings were taken with a Delfin Vapometer in an environment with ~10-60% Relative Humidity (RH) and is at ~20-25°C. Readings for each zone were taken every 2 hours until 4 hours passed from initial application of the hydrogels - time stamps are as follows: 0 hours (baseline, before application), 2 hours, and 4 hours. Readings were taken in triplicate from different points in each zone. Readings were reported as TEWL (g/m2h). Percent reduction in TEWL (%) from baselines for each zone were then calculated, and average percent reduction in TEWL (%) for each zone was reported. Greater average percent reductions in TEWL were associated with greater skin protective properties of the hydrogel. Neither the test administrators nor the subjects were blinded as the raw data was collected only from laboratory instruments.
Skin Protection of a hydrogel with GreenGard™ Land competitor.
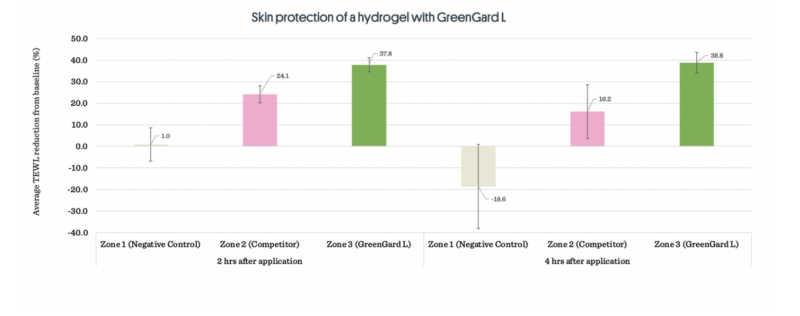
Figure 1: Average TEWL reduction for all three zones across all subjects. Zone 1 - Negatie control (no sample applied),
Zone 2 - Hydrogel 1 (with competitor), Zone 3 - Hydrogel 2 (with GreenGardTM L).CONCLUSION: The GreenGard™ L hydrogel showed not only consistent reductions of TEWL at both the 2 and 4 hour mark, but reductions higher than those of the competitor hydrogel. This could be explained by the arginine deposited at the skin surfact being not only a humectant, but also a precursor to nitric oxide, a molecule that plays multiple roles in wound healing and tissue repair1. As arginine is deposited onto and eventually penetrates into the skin, it would theoretically become available for keratinocyte nitric oxide synthase to initiate nitric oxide synthesis2.
- GreenGard™ L Collagen Upregulation Study
Objective: To asses the capacity of GreenGard™ L to stimulate the synthesis of collagen type I and collagen type III protein levels, after in vitro treatment in Normal Human Dermal Fibroblasts (NHDF).
Study Design: For cell viability assay, NHDF were cultured in the presence of GreenGard at different concentrations for 48 hours. After treatment, cell viability was quantified using MTT assay and the sub-cytotoxic dose range was characterized. For the protein synthesis study, NHDF were cultured during 48 hours in the presence of the sample at 0.001 %, 0.01 %, and 0.1 % (v/v). After treatment, secreted pro-collagen I (collagen type I alpha I) and pro-collagen III (collagen type III alpha I) levels were quantified by ELISA and normalized to cell viability.
Collagen I and Collagen III Data
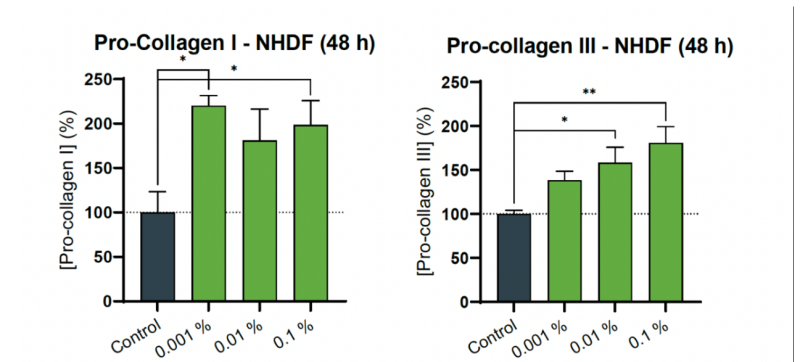
Figure 1: Effect of GreenGard L in synthesis of collagen type I alpha I and collagen type III alpha I protein levels. Bar graph representing secreted collagen type I alpha I and collagen type III alpha I protein levels relative to cell viability (resorufin), after 48 hours of treatment in Normal Human Dermal Fibroblasts with GreenGard at 0.001 %, 0.01 %, or 0.1 % concentrations (v/v). Asterisks (*) over bars indicate statistical significance (ordinary one-way ANOVA test followed by Dunnett’s post hoc multiple comparisons test).
CONCLUSION: The in vitro treatment for 48 hours in Normal Human Dermal Fibroblasts with “GreenGard L Batch FM-121120.1” at sub-cytotoxic concentrations displays antiaging and firming effects, substantiated in a significant increase of collagen type I and collagen type III levels, compared to the untreated control.
- GreenGard™ L & Sodium Benzoate System - PCPC Preservative Adequacy Test (wet wipe , pH 4.68)
Testing Report Wet Wipe Solution Challenge
GreenGard™ Lhad its preservation properties tested in a wet wipe formula (an O/W emulsion with an oil phase comprising 1.75% of the formulation. A preservative system of 0.90% of formula w/ GreenGardTM L and 0.35% of formula w/ Sodium Benzoate was tested at formula pH of 4.68. A 28-day PCPC Preservative Adequacy Test was performed, in which the formula was challenge tested against Gram (+) & Gram (-) bacteria, yeast, and mold inoculations. Recommended minimum criteria was a 99.9% reduction in bacterial colony forming units cfu/mL and a 90% reduction in yeast and mold cfu/mL within 7 days of inoculation, with reduction maintained for the remainder of the trial.
Microbial species used for the challenge testing listed below:
Gram (+):S. aureus
Gram (-):P. aeruginosa, E. coli, B. cepacia
Yeast: C. albicans
Mold: A. brasiliensis
RESULTS: Sample met the criteria for the preservative adequacy test. The formula showed a 99.99% reduction in cfu/mL within 1 day of inoculation for the Gram (+) & (-) bacteria and yeast, and within 7 days of inoculation for mold.GreenGard™ L & Sodium Benzoate System - PCPC Preservative Adequacy Test (wet wipe , pH 4.68)
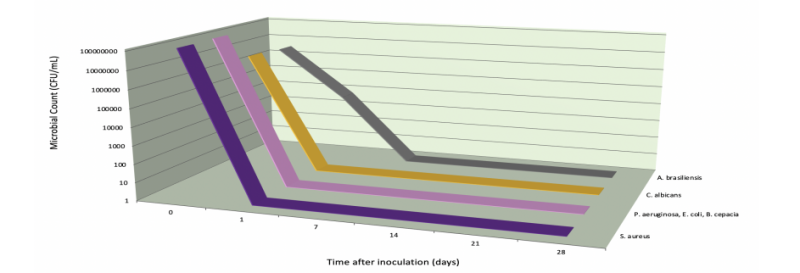
Figure 1: Figure 1 - GreenGard™ L (0.90%) & Sodium Benzoate (0.35%) reduction of cfu/mL against bacteria, yeast and mold. Total kill was observed for bacteria and yeast after 1 day, and was observed for mold after 7 days. However, a 99.82% reduction in cfu/mL for mold was still observed after 1 day. Reduction maintained for remainder of trial.
CONCLUSION: A preservative system of GreenGard™ L at 0.90% and Sodium Benzoate at 0.35% in a mostly aqueous O/W emulsion (pH 4.50 - 5.00), is a broad-spectrum, germicidal preservative. This system is highly effective against Gram (+) & (-) bacteria and yeast, and effective against mold. It is recommended that GreenGard™ L be used in conjunction with an antifungal agent in formulation to achieve broad-spectrum preservation.
Packaging & Availability
Storage & Handling
- Shelf Life
- 12 months
