Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Manufactured By
- Plastics & Elastomers Functions
- Technologies
- Product Families
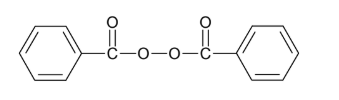
- Chemical Structure
Features & Benefits
- Materials Features
Applications & Uses
- Compatible Polymers & Resins
- Use Level
- 0.7 - 1.4
- Application Information
Perkadox L-50S-ps is mainly used for the crosslinking of silicone rubbers.
• Perkadox L-50S-ps can easily be incorporated into a silicone rubber compound on a 2-roll mill.
• Safe processing temperature: 85°C (rheometer ts2 > 20 minutes).
• Typical crosslinking temperature: 105°C (rheometer t90 about 12 minutes).
Properties
- Physical Form
- Appearance
- White homogeneous paste
- Typical Properties
Value Units Test Method / Conditions Active Oxygen Peroxide Content 6.61 % - Self-Accelerating Decomposition Temperature 60 °C - Storage Temperature max. 30 °C - Particle Size max. 50 μm - Water Content max. 1.0 % - Inorganic + Organic Hydrolysable Chloride max. 0.15 % - Assay Content 49.0 - 51.0 % - Actual Product 3.24 - 3.37 % - Molecular Weight 242.2 - - - SDS Physical and Chemical Properties
Value Units Test Method / Conditions Organic Peroxides 50 % - Active Oxygen Content 3.3 % - Oxidising Properties Not classified as oxidising. - - Explosive Properties Not Explosive - - Kinematic Viscosity Thixotropic - - Dynamic Viscosity (at 20°C) Thixotropic - - Self-Accelerating Decomposition Temperature 70 °C - Decomposition Temperature SADT - (Self accelerating decomposition temperature) is the lowest temperature at which self accelerating decomposition may occur with a substance in the packaging as used in transport. A dangerous self-accelerating decomposition reaction and, under certain circumstances, explosion or fire can be caused by thermal decomposition at and above the SADT. Contact with incompatible substances can cause decomposition below the SADT - - Soluble In Most organic solvents - - Insoluble in (at 20°C) Water - - Relative Density (at 20°C) 1.12 - - Flammability (Solid, Gas) Decomposition products may be flammable - - Flash Point Above the SADT value - - Boiling Point Decomposes below the boiling point - - Melting Point Decomposes before melting - - pH Value Weakly Acidic - - Odor Faint - - Appearance White paste - - - Characteristics
Value Units Test Method / Conditions Density (at 20°C) 1.12 kg/m³ - - Thermal stability
- Organic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition.
- The lowest temperature at which self- accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT).
- The SADT is determined on the basis of the Heat Accumulation Storage Test.
Regulatory & Compliance
Packaging & Availability
Storage & Handling
- Shelf Life
- 6 months
- Storage Information
- Due to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time.
- To minimize the loss of quality, AkzoNobel recommends a maximum storage temperature (Ts max.) for each organic peroxide.
- When stored under these recommended storage conditions, Perkadox L- 50S-ps will remain within the AkzoNobel specifications for a period of at least six months after delivery.
- Storage and Handling Information
- Keep containers tightly closed. Store and handle Perkadox L-50S-ps in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room.
- Avoid contact with reducing agents (e.g. amines), acids, alkalis and heavy metal compounds (e.g. accelerators, driers and metal soaps).
