Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Manufactured By
- Industrial Additives Functions
- Plastics & Elastomers Functions
- Molecular formula
- Al(OH)₃
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- Industrial Additives Features
- Materials Features
Applications & Uses
Properties
- Physical Form
- Appearance
- Crystalline powder
- Typical Physical Properties
Value Units Test Method / Conditions Particle Size (100 mesh) 0 % - Particle Size (200 mesh) 0.7 % - Particle Size (325 mesh) 14 % - Bulk Density (Loose) 57 lb/ft³ - Packed Bulk Density 69 lb/ft³ - Median Particle Size 10.0 microns - Oil Absorption 35 ml/100g - Decomposition Temperature 428 °F - Refractive Index 1.57 - - Specific Gravity 2.42 - - Mohs Hardness 2.3 - 3.5 - - - Chemical Composition
Value Units Test Method / Conditions Loss on Ignition (at 1000°C) 34.5 % - Free Moisture Content max. 0.3 % - Ferric Oxide Content 0.02 % - Silicon Dioxide Content 0.02 % - Total Sodium Oxide Content 0.3 % - Aluminum Hydroxide Content 99.5 % - - SDS Physical and Chemical Properties
Value Units Test Method / Conditions pH Value (at 20°C saturated solution) 8 - 9 - - Water Solubility (at 20°C) 0.00009 g/L - Decomposition Temperature min. 200 °C - Boiling Point 2980 °C - Relative Density 2.42 - - Soluble in Sulfuric acid - - Soluble in Alkaline aqueous solutions - - Soluble in Hydrochloric acid - - Soluble in Other strong acids in the presence of some water. - - Melting Point Decomposes before melting - - Auto Ignition Temperature Does not ignite - - Oxidizing Properties None - - Physical State Powder - - Appearance White odorless powder - -
Regulatory & Compliance
Technical Details & Test Data
- Product Properties
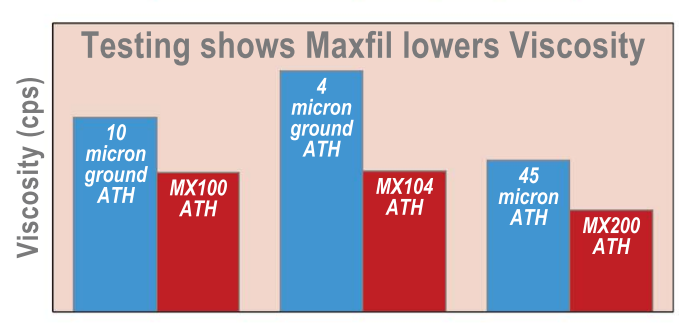
.Testing shows MX100 results in a 28% decrease in viscosity over a 10 micron ground ATH. Designed for use in highly filled resin systems requiring a 10 micron ATH. MX100 particle size distribution is tightly controlled to achieve high filler loadings and at the same time keep the working viscosities of the compound low.